Abstract
Introduction: The emergence of novel agents (NAs) in chronic lymphocytic leukemia (CLL) management marks a move away from a chemotherapy-based paradigm in less than a decade. This has resulted in better treatment tolerance and improved overall survival. The Veteran Health Administration (VHA) offers an attractive population for examining CLL treatments patterns because of two main reasons. It serves mainly older white male population, where incidence of CLL is higher, and is the only single payer national health program that provides equal access to medications. This aim of the study is to assess large population-based uptake of the NAs and to analyze the trends in chemotherapy-based regimen replacement by NA in front-line (1L) CLL.
Methods: This is a retrospective study of structured EMR data for adult patients with ICD9 and ICD10 CLL codes within the VHA who initiated 1L treatment with one of 14 CLL treatment [eight NAs and six chemotherapy-based regimens] from 1/1/2014 to 12/31/2021. Data were extracted from the VHA electronic health record. Patients came from all 18 Veterans Integrated Service Networks, spanning all 50 states and US territories. Descriptive statistics were used to summarize baseline characteristics and treatments by calendar year.
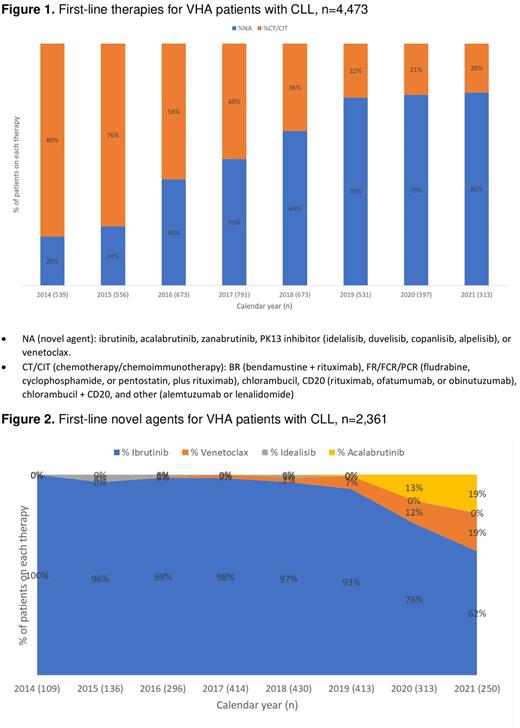
Results: A cohort of 4,473 patients were identified who received at least one 1L CLL therapy of interest. The median age was 69 years and 98% were male. The proportion of patients who received an NA as 1L treatment for CLL increased from 2014 to 2021, with a corresponding decrease in chemotherapy-based regimens (Figure 1). In 2017, the proportion of NAs surpassed chemotherapy-based regimen use (52 vs 48%, respectively). This gap continued to widen throughout the study period, with the use of chemotherapy-based regimens plateauing for the last three fiscal years of the study period at ~20%. The use of ibrutinib (90%) accounted for the greatest proportion of 1L NA use over the study period, followed by venetoclax (5%), acalabrutinib (4%), and idelalisib (1%). Until 2018, ibrutinib made up nearly 100% of the NA use with a steady decline thereafter reaching 62% in 2021. The proportion of patients on 1L venetoclax increased annually from 2018 (2%) to 2021 (19%). Similarly, the proportion of patients on 1L acalabrutinib increased annually from 2019 (0%) to 2021 (19%). The proportion of patients on 1L idelalisib increased early in 2015 (4%) but declined throughout the remaining study period (0-1%) (Figure 2).
Conclusions: This is one of the largest studies that provides important epidemiological data regarding emerging 1L treatment patterns among VHA patients with CLL. There has been a major shift in 1L treatments with NAs outpacing chemotherapy-based regimens from 2017-2021. Within NAs, there is an ongoing transition from first generation Bruton Tyrosine Kinase (BTK) inhibitor to fixed duration treatments and second generations BTK inhibitors with better side effect profiles. The trend shift will continue to evolve as multiple NAs combinations that offer deeper response and limited/guided treatment duration enter the treatment landscape.
Disclosures
Frei:AstraZeneca: Research Funding. Ryan:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Nooruddin:Astrazeneca: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal